
Table of Contents
Aluminum Air Battery in Electric Vehicles
What if we don’t have to plug our electric vehicle into an electrical socket for charging? What if there was a much lighter, cheaper, and greener battery that uses just air + water and metal aluminum to generate electricity?
and what if does the job more efficiently by giving more than twice the driving range than a basic lithium battery in an electric car?
What is the solution?
The Aluminum Air Battery – an EV battery that does not require charging and provides long-range lightweight, cost-efficient recyclable, and ethically sourced batteries, is arguably the holy grail of the EV market.
- Long-range
- Lightweight
- Cost-efficient
- Recyclable
Although Lithium-ion batteries are revolutionizing the electric vehicles Market. but we typically see it gives a range of 300 miles or less which may not be enough for longer trips with Electric Cars.
And the major downside of a lithium-ion battery is that it cannot be recharged if the battery is completely discharged. Again lithium battery risk such as mechanical stress and battery degradation from temperature variation leakage due to overcharging and discharging.
These issues have left the door open for researchers and companies around the world to find better alternative battery technologies for electric vehicles.
Thus began the aluminum battery,
Oil marketing giant Oil Corporation of India and Israeli clean energy startup PHINERGY, who are working on commercializing aluminum-air batteries for electric vehicles in India.

What is an aluminum-Air Battery?
Aluminum air batteries generate electricity by reacting oxygen in the air with aluminum. The formula is quite simple: Aluminium, + Air, = Power. The reaction of oxygen and aluminum in the air produces electricity and has a charge that can be harnessed, they have the highest energy density of all batteries.
How does an Aluminum-Air Battery work?
The ‘fuel’ is an aluminum metal known as the anode, which reacts with the oxygen around it known as the cathode to create power.
Since the cathode is just oxygen from the surrounding air, there is no need to carry the weight of another metal like a conventional battery, and this makes it significantly lighter.
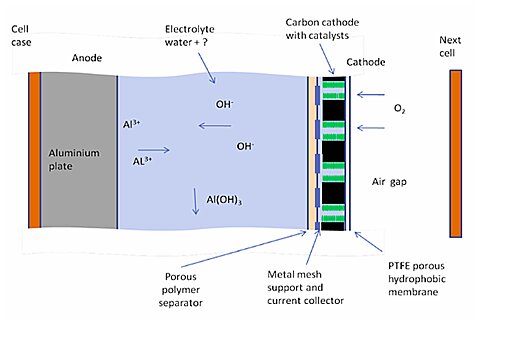
Air is sucked into the cathode system in which oxygen molecules are separated from the air and other components of the air by a silver-based catalyst to trigger a catalyst-containing chemical reaction that prevents CO2 from entering the battery.
This allows oxygen to pass through the electrolyte to react with water and produce negative hydroxide ions at the anode, positive ions of oxygen react with the aluminum to form aluminum trihydroxy-ide.
which is a white powder and electrons and it is these electrons that mean, it can generate electricity when the battery is connected to load like a light bulb these electrons move from anode to cathode to give back those electrons that were lost during the chemical reaction.
Advantages of aluminum-Air Battery Battery
- Aluminum Air Batteries do not require electricity as they do not require charging and thus are the biggest blessing for electric vehicles and its user.
- The aluminum hydroxide solution generated in the used battery can be sent to a recycling unit to get 100% aluminum back. Aluminum air batteries are 100% recyclable and thus safe for the environment.
- Aluminum air battery technology is safer because it uses only a water-based electrolyte that is free of toxins, as opposed to lithium-ion technology which uses highly flammable organic toxins-based electrolytes. and in a result, Aluminum air batteries are EV Thermal Friendly.
Disadvantages of Aluminum-Air Battery
- These batteries have major disadvantages and challenges like they are not rechargeable.
- Once the aluminum anode is consumed by its reaction with atmospheric oxygen, the battery will no longer produce electricity. It just stops working and has to be replaced.
- An aluminum-air battery would have to be taken to a battery swap station which is a big Disadvantage.
FAQs- Frequently Asked Question
What is an Aluminum air battery?
It’s a battery that uses aluminum alloy plates as anode water as electorate and an air electrode as a cathode.
How Does an Aluminum air Battery Work?
- The air from the atmosphere gets sucked into the air cathode system, which contains a catalyst.
- oxygen gets separated from the air and reacts with water to generate hydroxide(OH+) ions
- At the anode, which is made of pure virgin aluminum plate, the ionic oxygen (O+) reacts with aluminum trihydroxy-ide [ AI(OH)2].
- In this chemical reaction, electrons are released, which is not but energy or electricity.
Manufacturers in India
Indian Oil Corp has teamed up with startup Phinergy Ltd to develop an Israeli company’s aluminum-air battery in India.






Great information on all the research needed before these things are practical. Be sure to let our national leaders know so that they can hurry up and take all of our current energy supplies away so that “someday” we might be able to travel, stay warm and cook our food again!
I have been following the developments in aluminum air batteries now for at least 40 years. I was a physicist at the Lawrence Livermore National Laboratory during the early 1980’s when I attended seminars on this subject. As I recall, their research effort was transferred to a Chevron research lab in California- about which there were two theories:
1. Chevron slow walked the research because they didn’t want competition for their carbon based fuels they sell?
or
2.Chevron wanted to perfect the battery for the day the battery would be more profitable than the fuels they were selling?
or
3. Both?
Greetings.So far the major problem is that the Aluminium -Air battery is not rechargeable. Therefore the answer is to make it rechargeable. This can be achieved by having more than one cell in the circuit the extra cells not working but come into service one after another as the preceding cell becomes discharged. Provision must made to heat the dissolved anode (Aluminium) for reuse after it is dried up.In a circuit of the drying up cell D, the working cell W and the standby cells S,the Alumium-Air cells will provide electricity nonstop!
I enjoy, result in I found exactly what I used to be looking for.
You haᴠe ended my four day lengthy hunt! Ԍod Bless you man. Have a great day.
Bye