Battery-powered Vehicles (BEVs or EVs) are growing much faster than conventional Internal Combustion (IC) engines. This is because of a shortage of petroleum products and environmental concerns. EV sales have grown by 62 % globally in the first half of 2022 as compared to the first half of 2021.
Every Country and even car manufacturer has planned to switch to EVs/PHEVs, for example, the Indian government has set a target to achieve 30 % of EV car selling by 2030 and General Motors has committed to bringing new 30 electric models globally by 2025 respectively. Major car manufacturers are Tesla, Nissan, Hyundai, BMW, BYD, SAIC Motors, Mahindra Electrics, and Tata Motors.
The success of electric vehicles depends upon their Energy Storage Systems. The Energy Storage System can be a Fuel Cell, Supercapacitor, or battery. Each system has its advantages and disadvantages.
A fuel cell works as an electrochemical cell that generates electricity for driving vehicles. Hydrogen (from a renewable source) is fed at the Anode and Oxygen at the Cathode, both producing electricity as the main product while water and heat as by-products.
Electricity produced is used to drive the propulsion system of the vehicle.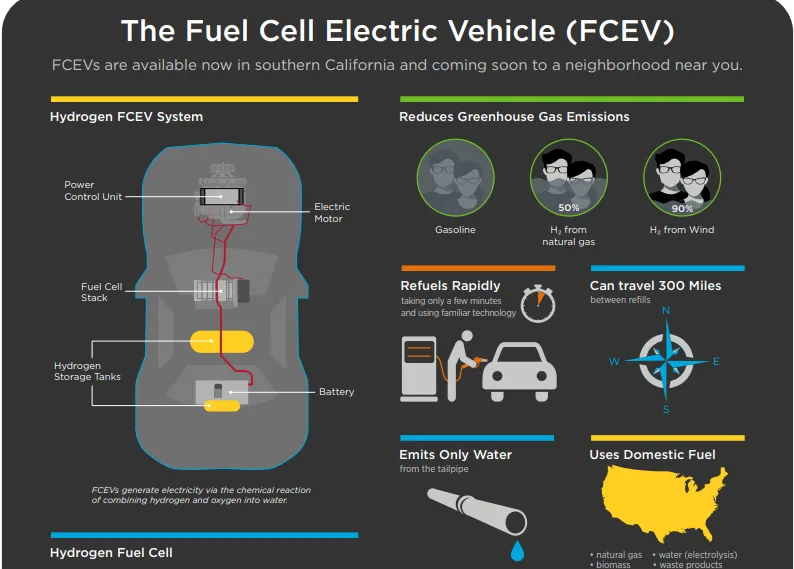
Advantages
Major car models using Fuel cells are Toyota Mirai (range up to 502 km), Honda Clarity (up to 589 km), Hyundai Tucson Fuel Cell (up to 426 km)
A supercapacitor (sometimes Ultra-Capacitor) is the same as a battery that can store and release electricity. In a supercapacitor, no chemical reaction happens rather than charge is stored statically.
It has also all the components like battery i;e.,
Supercapacitor use in the vehicle is projected to reach $593.6 million by 2026.
Some of the properties of a Supercapacitor are as follows:
| Property | Supercapacitor |
| Charge – time | 1–10 s |
| Cycle-Life | 500,000–1 million |
| Cell- Voltage | 2.3 to 3.0 V |
| Specific energy (Wh/kg) | 5–15 |
| Specific power (W/kg) | Max around 40,000 |
| Cost in USD per kWh | $8,000–$10,000 (large system) |
| Cost in USD per kW | $8–12 |
As no chemical reaction is involved in a Supercapacitor for storing electric charge, it can be charged or discharged within some seconds giving very high Power density and low Energy density among all other storage systems.
Because of its properties, Supercapacitor is used as an auxiliary storage system in the EVs/PHEVs and also to store energy during regenerative braking.
Supercapacitor electric buses are very common in China. Sunwin (a joint venture of Volvo and SAIC) brought SCs electric buses with the autonomy of 3 to 6 km. Buses are charged at each bus stop with a pantograph.
The major problems associated with using Supercapacitors in EVs are
The battery is the most commonly used in present-day EVs. It converts the electrochemical energy into electrical energy. Li-ion battery is very promising for EVs as compared to the Lead-acid battery, the nickel-cadmium battery (Ni-Cd), and the Nickel-Metal Hydride battery (Ni-MH).
This battery is the first commercial secondary battery that dominated the market for more than a century. In this battery lead and lead oxide are converted to lead sulfate. Sulphuric acid which is the electrolyte in this battery acts as a reactant and ionic transport carrier.
The lead-acid battery does not have good energy density so it is mainly used as an auxiliary battery in vehicles to power the internal circuit and to start the motor(starter) of vehicles.
Since this battery has been in use for more than 150 years, the technologies involved are matured and up to 98% of this battery is recycled.
Nickel-cadmium battery has comparatively more energy density than Lead-Acid battery. The anode is made up of Nickel and the cathode is made up of Nickel-oxide and an aqueous alkali solution is used as an electrolyte.
But Ni-Cd has a memory effect means it does not fully discharge itself and Cadmium is a toxic metal. It is no longer in use.
Ni-MH batteries have 2-3 times more energy density than Ni-Cd. The positive electrode mainly consists of nickel hydroxide as active material, the negative electrode consists of hydrogen-absorbing alloys, the alkaline electrolyte is used and the separator is made of fine fibers.
This battery has been used in Toyota Pyrius, Honda Insight.
Ni-MH battery has
Li-ion battery is the most widely used battery in Electric vehicles. Its unique features make it different from the other secondary batteries as it has
Li-ion battery is used by almost all major OEMs of EVs Tesla, Tata Motors, Volkswagen
Read More: Top 9 Lithium-ion Battery Manufacturers in India
Based on the electrode materials used in Li-ion battery, it has different properties exhibition:
For Negative electrode-When Graphite (Carbon) is used as the negative electrode, it can store one Li-ion per C- atom, when Silicon is used it can store four Li-ion per atom while when Lithium titanate oxide (LTO) is used life of the battery is increased
For Positive Electrode– When Lithium cobalt oxide (LCO) is used for portable devices but Co is toxic and expensive.
Nicol cobalt manganese (NMC) has good energy density and gives a good range to EVs but it is not thermally stable.
Presently in EVs, mainly LPF and NMC Li-ion batteries are used.
A brief pictorial representation of various types of Li-ion batteries indicating their properties suitable for
Source- ResearchGate
There are some problems also associated with using Li-ion batteries like
Solid-state batteries and metal-air batteries are some other batteries that are being looked at to overcome the problem associated with the discussed batteries.
A brief comparison of different battery technology properties:-
| Properties | Li-ion | Ni-MH | Ni-Cd | Lead-Acid |
| Cell Voltage (in V) | 3.6-4.2 | 1.2 | 1.2 | 2.1 |
| Self-Discharge(%) | 0.35–2.5 | 13.9–70.6 | 10 | 3–20 |
| Energy Density(Wh/kg) | 100–265 | 60–120 | 40–60 | 30–40 |
| Power Density(W/kg) | 250–340 | 250–1000 | 150 | 180 |
| Cycle life | 400–1200 | 180-2000 | 2000 | <1000 |
| Cost (USD/Wh) | 0.9361 | 0.8546 | 2.6778 | 0.69750 |
Read More:- Pros and Cons of Battery Swapping: An Energy-Efficient Solution
The unveiling of Maruti Suzuki's eVitara at the recently held auto show 2025 has generated a lot of excitement and…
Mahindra is all set to expand its EV portfolio in India with the debut of the XUV 3XO EV, as…
VinFast, the Vietnamese EV manufacturer, made its debut in the Indian EV market by announcing the launch of its two…
Godawari Electric Motors Pvt. Ltd., a leading electric vehicle manufacturer, today unveiled new additions to its product portfolio at the…
The future of urban mobility is electric, and two industry giants are leading the charge. Hyundai and TVS Motor Company…
TVS Motor Company today unveiled its futuristic vision for electric mobility at the Bharat Mobility Expo 2025, showcasing the iQube…
This website uses cookies.