 Li-ion batteries, which are exclusively utilized in mobile phones and laptop computers, are the driving force behind the digital electronic revolution in today’s mobile society. Commercial Li-ion battery success in the 1990s was the result of years of rigorous study and contributions from many brilliant scientists and engineers.
Li-ion batteries, which are exclusively utilized in mobile phones and laptop computers, are the driving force behind the digital electronic revolution in today’s mobile society. Commercial Li-ion battery success in the 1990s was the result of years of rigorous study and contributions from many brilliant scientists and engineers.
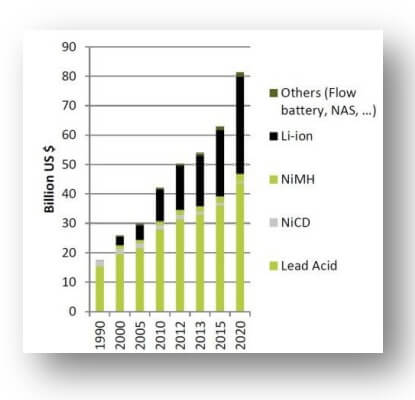
Many attempts have been devoted to boosting the effectiveness of Li-ion batteries, although remarkable progress has been made. To fulfill the growing need for energy storage, notably from increasingly popular electric vehicles, more research is needed to develop next-generation Li-ion batteries with drastically enhanced specific energy and volumetric energy density, cyclability, charging rate, stability, and safety.
Table of Contents
Lithium-ion Batteries
The development of next-generation Li-ion batteries still faces significant obstacles. To get beyond Li-ion batteries in the future, new battery designs must be further developed. The goal of this tutorial review is to teach the fundamental concepts, highlight current advances, and analyze the issues that Li-ion batteries face.
Fundamentals
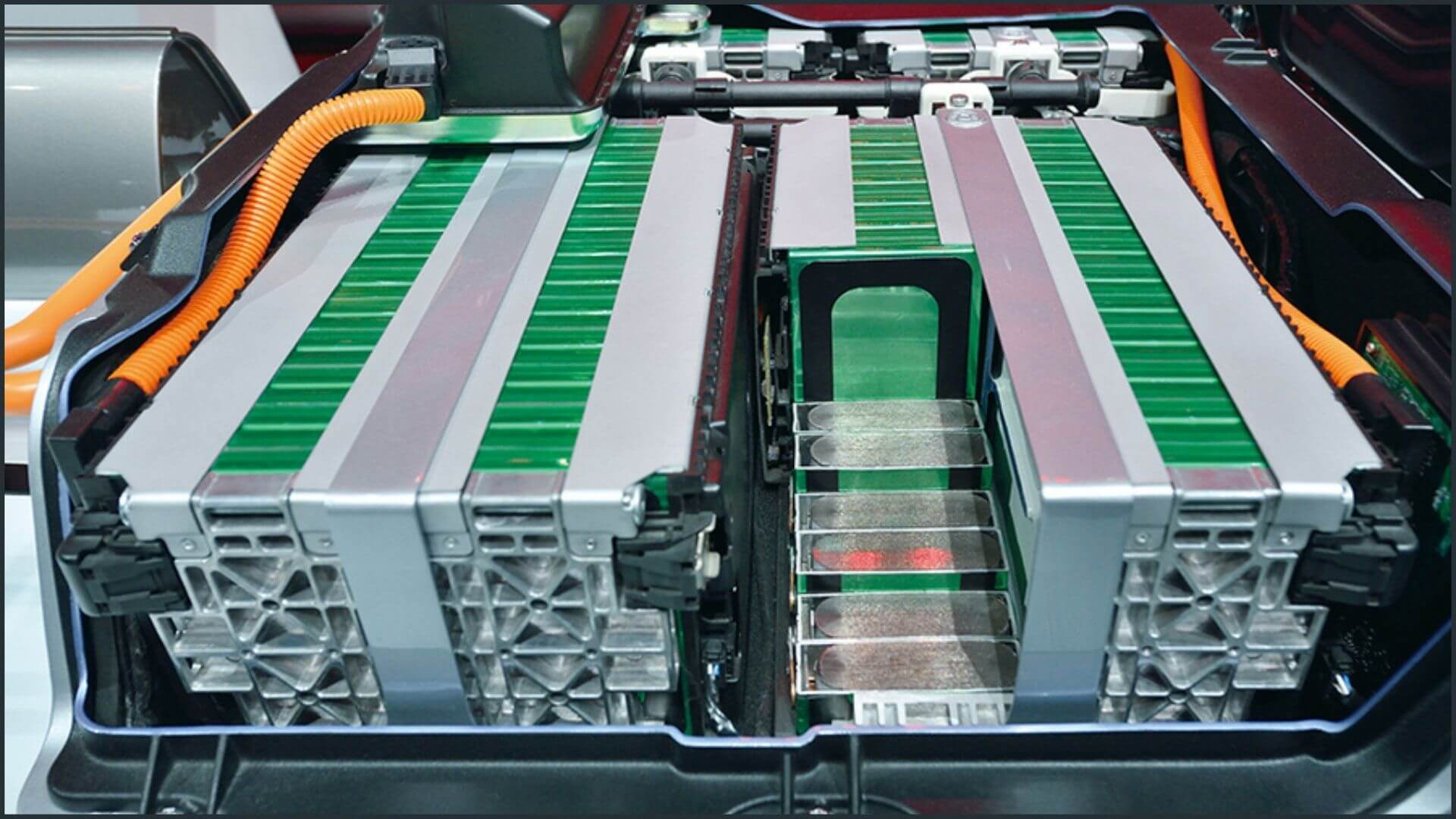
A Li-ion battery is made up of basic Li-ion cells that are connected in parallel, series, or mixed configurations to enhance current, voltage, or both. A module can have many battery cells. Multiple modules can make up a battery pack. The 85 kWh battery pack in a typical Tesla car, for example, contains 7104 cells.
A cathode (positive electrode) and anode (negative electrode) in contact with a lithium-ion electrolyte make up a basic Li-ion cell.
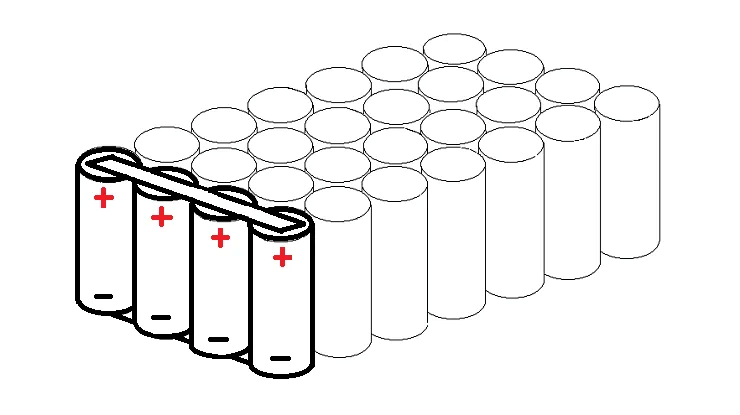
In a 7S/4P pack, four cells are connected in parallel (28 cells). On the top and bottom of these four cells, there is a full-length electrically connected metal strip (bus). The four parallel cells can be constructed in any configuration, but a straight line is the most straightforward way to grasp it.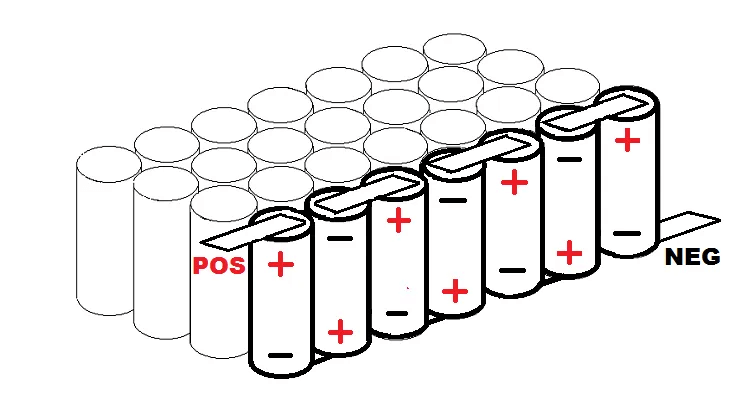
In a 7S/4P pack, there are seven cells in series with a nominal voltage of 24V. When completely charged to 4.1V per cell, this is 28.7V.
The electrodes are separated from one another by a separator, usually, a microporous polymer membrane, which allows lithium ions but not electrons to pass between them. Polymer, gel, and ceramic electrolytes, in addition to liquid electrolytes, have been investigated for use in Li-ion batteries.
A typical Li-ion battery cell’s basic operating mechanism is depicted in the Figure below.
Although several types of electrode materials, electrolytes, and separators have been researched, the basic design of Li-ion cells today is still the same as those Sony sold decades ago.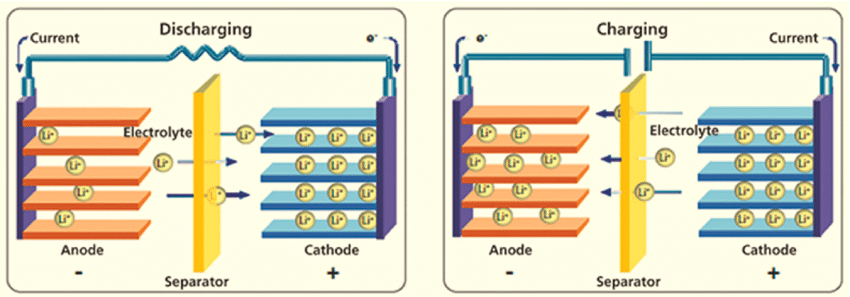
Commercial cells are typically built in a discharged state. The discharged cathode and anode materials (for example, LiCoO2, and LiFePO4) are stable in the atmosphere and can be easily handled in industrial practices. Yoshino made a significant contribution to the commercial production of Li-ion batteries by using discharged electrode materials in entire cells for the first time.
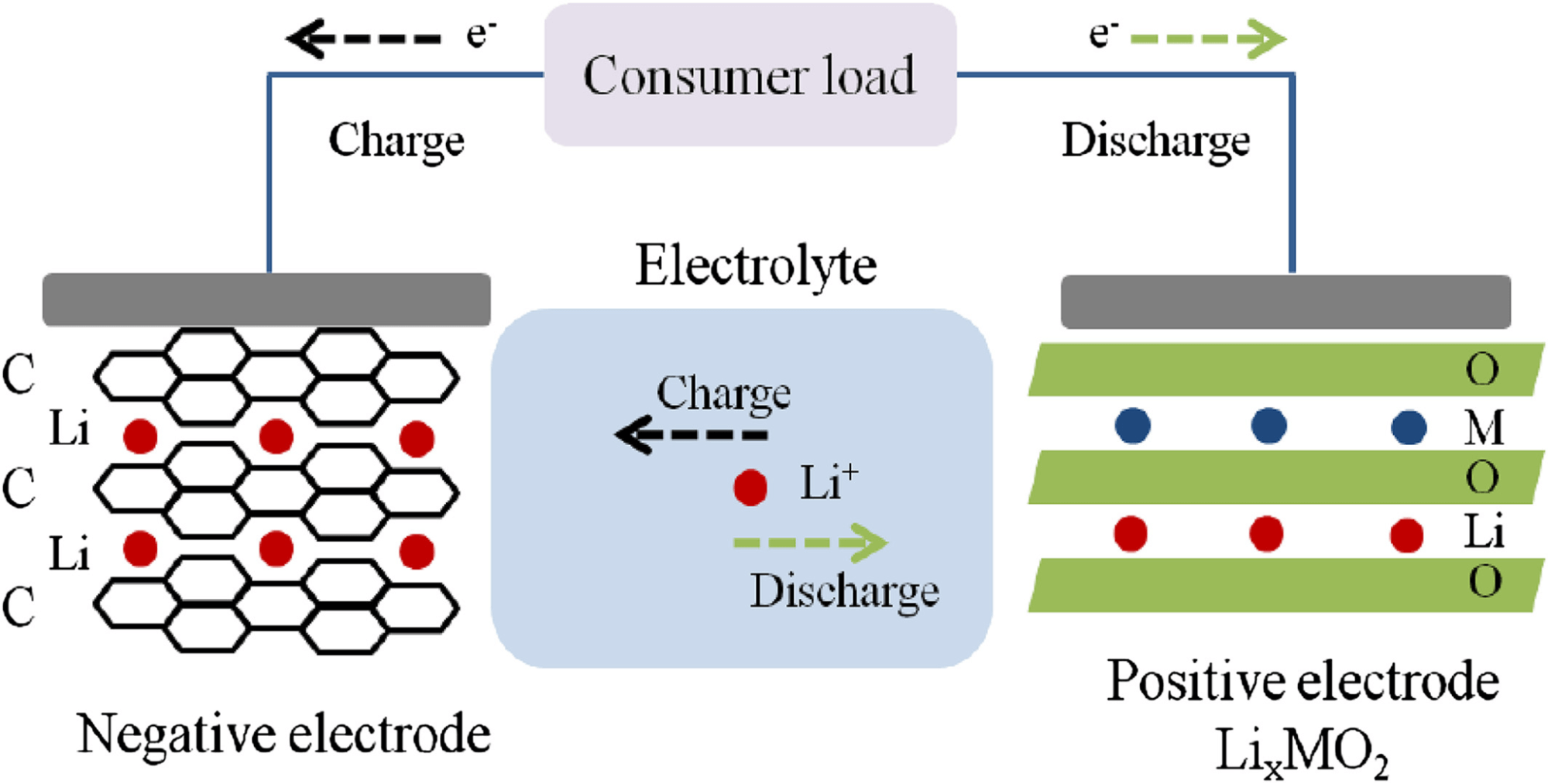
The two electrodes are externally connected to an external electrical supply during the charging process. The electrons are driven to leave the cathode and travel to the anode externally. Simultaneously, lithium ions travel from the cathode to the anode via the electrolyte in the same direction.
External energy is stored electrochemically in the battery as chemical energy in the anode and cathode materials, which have different chemical potentials. During the discharging process, electrons travel from the anode to the cathode via the external load to perform the work, while Li-ions travel from the anode to the cathode in the electrolyte.
During charge and discharge cycles, Li ions shuttle between the anode and cathodes, which is known as the “shuttle chair” mechanism.
The rate of charge or discharge, often known as the C-rate, is a measurement of how quickly a battery can be charged and drained. The battery is entirely depleted at 1 C, with maximal capacity released in 1 hour. Li-ion batteries with carbonaceous anodes, which are often used in personal mobile devices, take 1–4 hours to fully charge.
Li-ion batteries used in electric vehicles may take much longer to fully charge, such as overnight, while they can be charged quickly to particular low SOCs at high currents using special charging methods.
One of the most active research directions in the Li-ion battery community is to improve the rate of performance so that the time required to charge a battery may be drastically decreased, which is critical for the commercial acceptability of electric vehicles.
Technology Advances
Recently, efforts have been made to use nanostructured materials for Li-ion batteries as an alternative electrode material. It is widely assumed that the nanoscale size and shape tunable properties of those lithium-active materials can provide additional parameters for further optimizing their electrochemical performances.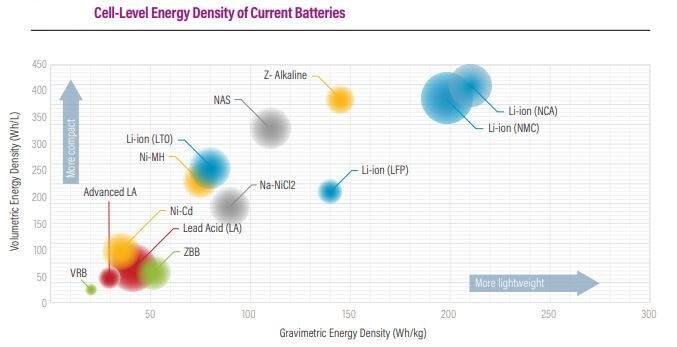
Lithium-ion batteries are available in a variety of shapes and sizes, and they are not all created equal. The descriptions of six different lithium-ion battery types, as well as their compositions and common applications, are provided below:
1. Lithium Iron Phosphate (LFP)
FeLiO4P
In lithium-iron phosphate batteries, often known as li-phosphate batteries, phosphate is employed as a cathode. Their low resistance properties improve their thermal stability and safety.
- Nominal voltage : 3.2V
- Cycle: 2000 +
- Applications: Electric vehicles, drones, power backup.
2. Lithium Cobalt Oxide (LCO)
LiCoO2 or CoLiO2
Lithium cobalt oxide batteries, commonly known as lithium cobaltate or lithium-ion cobalt batteries, are made from lithium carbonate and cobalt. Because of their high specific energy, these batteries are employed in cell phones, laptops, and electronic cameras. Lithium ions move from the anode to the cathode during discharge, with the flow reversing when the battery is charged. They have a cobalt oxide cathode and a graphite carbon anode.
- Nominal voltage : 3.6V
- Cycle: 1000 +
- Applications: Mobile phones, tablets, laptops, and cameras.
3. Lithium Manganese Oxide (LMO)
LiMn2O4
Lithium manganese oxide batteries are also known as lithium manganese batteries, lithium-ion manganese batteries, li-manganese batteries, and manganese spinel batteries. This type of battery technology was first found in the 1980s, with the first publication in the Materials Research Bulletin in 1983. In 1996, Moli Energy produced the first commercial lithium-ion batteries with a lithium manganese oxide cathode.
- Nominal voltage : 3.6V
- Cycle: 1000 +
- Applications: Portable power tools, medical instruments, and some hybrid and electric vehicles.
4. Lithium Nickel Manganese Cobalt Oxide (NMC)
LiNiMnCoO2
NMC batteries, also known as cobalt oxide batteries, are made up of a range of materials that are used in lithium-ion batteries. A cathode made of nickel, manganese, and cobalt is also provided.
Like other lithium-ion battery types, NMC batteries can have a high specific energy density or specific power. They cannot, however, have both characteristics. The most popular applications for this battery are power tools and car powertrains.
- Nominal voltage : 3.7V
- Cycle: 500- 1000
- Applications: Power tools as well as electric powertrains for e-bikes, scooters, and some electric vehicles.
5. Lithium Nickel Cobalt Aluminium Oxide (NCA)
LiNi0.8Co0.15Al0.05O2
NCA batteries aren’t extensively employed in consumer electronics, they have a lot of promise in the automotive industry. NCA batteries provide a high-energy option with a long lifespan, but they are less safe and more expensive than other lithium-ion battery types. NCA batteries must be complemented by monitoring systems to assure driver safety.
- Nominal voltage : 3.6V
- Cycle: 2000 +
- Applications: In-grid storage and electrical power terrain applications, NCA is the battery of choice for Tesla.
6. Lithium Titanate (LTO)
Li2TiO3
Finally, lithium titanate, or li-titanate, is a form of battery with an increasing variety of applications. The lithium-titanate battery has an extraordinarily fast recharge time due to its superior nanotechnology.
Electric vehicle and bicycle manufacturers use lithium-titanate batteries, and this type of battery has the potential to be used in public transportation electric buses.
These batteries, on the other hand, have a lower intrinsic voltage or energy density than other lithium-ion battery types, which could pose a challenge when it comes to powering autos efficiently. Regardless, lithium titanate batteries have a higher density than non-lithium-ion batteries, which is an advantage.
- Nominal voltage : 3.6V
- Cycle: 2000 +
- Applications: Electric vehicles and charging stations, uninterrupted power supplies, wind and solar energy storage, solar street lights, telecommunications systems, and aerospace and military equipment are just some of the use cases.
Li-S, Si-NMC, Solid-State Batteries, and PEM Fuel Cells
are among the technologies on the horizon.
Several other Li-ion battery technologies are currently being developed in addition to the ones mentioned above. The R&D community and industry are working on different elements of current battery technologies, as well as future battery technologies, to lower costs and enhance performance.
Four such innovative technologies are addressed in this part, together with a current assessment of their level of development, prospects, and obstacles.
Improving the volumetric (Wh/L) and gravimetric (Wh/kg) energy density of EVs is one of the key thrust areas of global R&D initiatives. The battery pack is tiny due to a combination of advancements in these two factors. A smaller battery pack allows for a larger battery pack to be installed in the vehicle, resulting in a greater driving range.
Attempts have been undertaken over the last few decades to boost the energy density of all storage methods. Initially, the main driving factor was for use in electronic gadgets, but in the last 20 years, the potential application in electric vehicles has supplied a huge drive.
Li-S
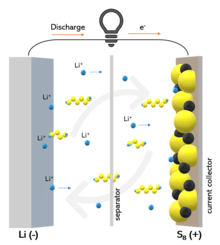
Due to its high capacity, low cost, abundance, and long-term sustainability, sulfur is a suitable cathode material for rechargeable lithium batteries. Under lean electrolyte conditions, however, lithium-sulfur batteries suffer from poor cycle life and low energy density due to the dissolution of lithium polysulfide intermediates.
The chemical bonding between sulfur and carbon/oxygen in an oxygen-rich dense carbon host can stabilize sulfur and improve the stability of lean electrolyte lithium-sulfur batteries. This research paves the door for the development of chemically stabilized sulfur compounds for lithium-sulfur batteries that are both stable and high-energy.
Si-NMC
Silicon (Si) is a popular anode material for lithium-ion batteries because of its high theoretical capacity and low cost. Si, on the other hand, changes volume by 300 percent during cycling, resulting in rapid capacity degradation.
The cycle life of Si anodes can be extended by using tiny Si particles in a flexible composite matrix. Stress-potential coupling is also seen in Si anodes, where the open-circuit voltage is affected by the applied stress.
Solid-State Batteries
Solid-state batteries use solid electrodes and a solid electrolyte rather than the liquid or polymer gel electrolytes seen in lithium-ion and lithium polymer batteries. Ceramics (e.g. oxides, sulfides, phosphates) and solid polymers have been proposed as solid electrolytes for solid-state batteries. Pacemakers, RFID, and wearable gadgets all require solid-state batteries. They may be safer since they have higher energy densities, but they come at a considerably higher price. Energy and power density, durability, material prices, sensitivity, and stability are all obstacles to wider use.
PEM Fuel Cells
PEMFCs (proton-exchange membrane fuel cells), also known as polymer electrolyte membrane (PEM) fuel cells, are a form of fuel cell that is primarily being developed for transportation uses, as well as stationary and portable fuel-cell applications.
Lower temperature/pressure ranges (50 to 100 °C) and a specific proton-conducting polymer electrolyte membrane distinguish them. PEMFCs produce electricity and work in the opposite direction of PEM electrolysis, which consumes it. They’re a strong contender to take over from the Space Shuttle’s aging alkaline fuel-cell technology.
Raw material for Battery Manufacturing
As the Lithium-ion Battery Market Advances, Raw Materials Become More Important.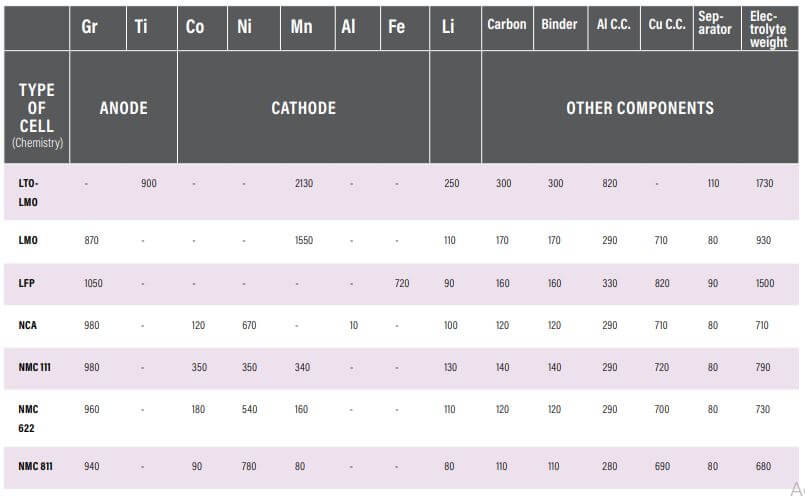
Manganese, nickel, cobalt, copper, graphite, and aluminum are among the major elements utilized in the electrode-making process in LIB.
Cobalt in the Cathode
Cobalt is a rare, poisonous, and shiny mineral found in the negatively charged electrode (or cathode) of practically all lithium-ion batteries currently in use. It’s pricey, heavy and related to unethical mining, dramatic price swings, and a shaky global supply chain. It’s no surprise that many battery makers want to get rid of cobalt. However, the substance is critical for stabilizing batteries and increasing their energy density.
Cobalt can make up a fifth of a lithium-ion cathode, which usually comes in one of two flavors: NMC (nickel manganese cobalt oxide) or NCA (nickel copper-cobalt oxide) (nickel cobalt aluminum oxide).
Anode

The negative terminal of a battery is the anode, which is always related to the release of electrons in the external circuit.
The anode is the positive pole of a rechargeable cell during charge and the negative pole during discharge. The use of high-capacity anode and cathode materials is a critical variable in the creation of a high-energy-density battery (a battery that can store more power in a given space per unit volume, such as lithium-sulfur batteries).
As a result, alkali metals are an excellent candidate for these battery systems.
Lithium

A lithium-ion (Li-ion) battery is a high-performance battery that employs lithium ions as a key component of its electrochemistry. Lithium atoms in the anode are ionized and separated from their electrons during a discharge cycle.
The lithium ions go from the anode via the electrolyte to the cathode, where they recombine with their electrons and become electrically neutral. Between the anode and the cathode, the lithium ions are tiny enough to pass through a micro-permeable separator.
Li-ion batteries can have a very high voltage and charge storage per unit mass and volume, thanks in part to lithium’s tiny size (third only to hydrogen and helium).
Nickel

Nickel (Ni) has been used in batteries for a long time, most notably in nickel-cadmium (NiCd) and the longer-lasting nickel-metal hydride (NiMH) rechargeable batteries, which first appeared in the 1980s.
Their use of power tools and early digital cameras demonstrated the possibility of portable technologies, transforming how we work and live. In the mid-1990s, the Toyota Prius was the first automobile to use NiMH batteries substantially.
Around the same time, the first commercial Li-ion battery applications appeared, first in camcorders and then in smartphones, laptops, and a slew of other portable gadgets that we now take for granted.
Electrolyte

The electrolyte is the medium that facilitates ion transport between a cell’s cathode and anode. Electrolytes are commonly conceived of as liquids containing dissolved salts, acids, or alkalis that are required for ionic conduction.
Separator: A battery separator is a form of polymeric membrane that sits between the positively and negatively charged anode and cathode. This placement aids in the prevention of electrical short circuits.
Manganese

Manganese, one of the most plentiful metals on Earth, might be used to replace costly cobalt in battery cathodes. Cobalt is used in some of the lithium-ion batteries that power today’s electric cars.
Cobalt is a blue-gray metal that helps a battery pack more power while remaining safe, but it comes at a cost: it’s pricey and often mined in dangerous areas. As the market for energy storage expands, researchers are looking for battery chemistries that use significantly less or no cobalt.
Aluminum

Aluminum has long been thought to be a better potential battery basis than lithium since it can exchange three electrons for each ion, compared to one for lithium, allowing for up to three times more energy density.
Graphite

Graphite, the principal material used for one of two electrodes known as the anode, is an important component of lithium-ion batteries. Lithium ions travel from the cathode to the anode when a battery is charged, passing through an electrolyte buffer that separates the two electrodes.
As the battery drains its energy, the process is reversed. While a variety of materials can be used for the cathode, most anodes employ graphite because of its abundance, low cost, and extended cycle life. The cycle life of a battery determines how long it can keep a charge and contributes to technological breakthroughs.
Copper
Copper is used in electric motors, batteries, inverters, wiring, and charging stations in electric vehicles. The stator windings of a pure electric car can contain more than a mile of copper wiring. The rising demand for copper will have a big impact on the market.
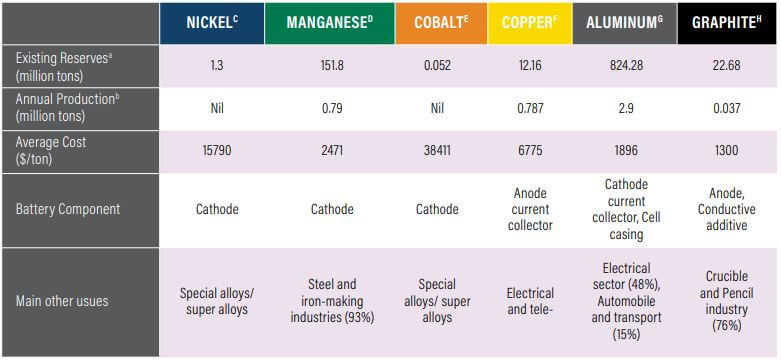
The Supply Chain of Raw Materials Used in the Manufacturing
The availability of key raw material reserves and annual production in India to support Li-Ion manufacturing.
Lithium-Ion Battery Challenges
Based on current battery chemistry, Li-ion batteries are considered relatively mature. Li-ion batteries have long been the standard in mobile electronic devices, such as cell phones and laptop computers, and are now beginning to play a larger part in electric cars. Lithium-ion batteries will be evaluated for use in sustainable energy systems to store energy generated from renewable sources.
The rising demand for energy storage necessitates further improvements to existing Li-ion batteries, as well as the development of next-generation Li-ion batteries, to lower Li-ion battery costs. The development of new battery chemistry to replace the existing Li-ion battery technology is still extremely difficult.
It is desirable to find electrode pairs with both high specific capacities and high operating cell voltage to boost the energy density of Li-ion batteries.
There is a slew of anode options that could drastically boost certain capacities, particularly those based on the highly appealing Si and Sn elements.
Preparing Silicon nanoparticles on a big scale at a reasonable cost remains a challenge. Thermal decomposition is a problem with Sn-based anodes, which results in poor cycling performance.


Another issue that needs to be addressed is the issue of safety. The news about Li-ion battery fires involving Boeing 787 passenger planes and Tesla Model S cars highlights the importance of battery safety. To ensure the wide acceptance of electric vehicles and to expand the market for Li-ion battery-powered vehicles, automakers should invest heavily in battery management systems to improve the safety of large battery packs in vehicles.
Furthermore, future battery research should consider the technologies’ life cycle assessment (LCA) to determine whether the batteries are truly green or not. Large amounts of contaminated waste and places will result from the mass production of Li-ion batteries for electric vehicles.
It has the potential to reduce agricultural productivity near mine sites, reduce air quality near processing facilities, and raise energy costs near factories.
Furthermore, additional fossil fuel consumption may be required to meet factory demand for Li-ion battery production.
The preparation of “nano” materials to increase the storage capacity of next-generation Li-ion batteries is gaining popularity around the world. Because the electrochemical properties of nanomaterials are size and shape-dependent, the development of nanomaterials could provide enormous opportunities.
In the last two decades, the term “nano” has gotten a lot of attention in the battery community. The challenging issue that is rarely mentioned is that the energy density (by volume) of “nano” materials is extremely low due to the low volumetric density of nanomaterials.
Summary
Almost everyone on the planet has been impacted by lithium-ion batteries. The commercial success of Li-ion batteries was the result of decades of intensive research and contributions from many great scientists.
Recently, much effort was expended to improve the performance of Li-ion batteries, with varying degrees of success. There are still significant issues. To develop next-generation Li-ion batteries, research must be intensified. Private companies are investing heavily in Li-ion battery research and development, which could result in incrementally advanced products with significant direct impacts on our society.
Academic sections could contribute by coming up with novel ideas and concepts.







Novyny